AQS Baden-Württemberg
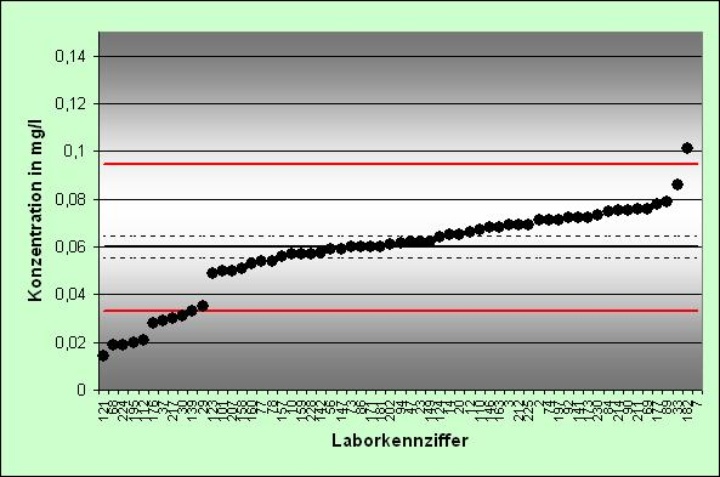
Das Institut für Siedlungswasserbau, Wassergüte- und Abfallwirtschaft führt unter dem Namen AQS Baden-Württemberg diverse Aktivitäten zu Qualitätssicherung in chemisch-analytischen Laboratorien durch. In erster Linie sind das Eignungstest-Ringversuche (hauptsächlich auf dem Gebiet der Wasseranalytik) und Lehrgänge für Laborpersonal. Daneben organisiert die AQS Baden-Württemberg gelegentlich auch Validierungsringversuche für neue Verfahren in der Wasseranalytik im Zuge der Normung durch ISO, CEN und DIN. Auf Wunsch werden auch individuelle Eignungsprüfungen angeboten, die Laboratorien im Rahmen der Akkreditierung nach DIN EN ISO/IEC 17025 benötigen.
Lehrgänge der AQS Baden-Württemberg
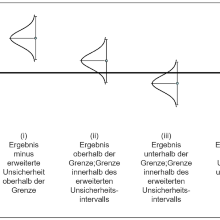
Die AQS Baden-Württemberg führt regelmäßig Lehrgänge zur Probenahme von Abwasser, zum Führen von Qualitätsregelkarten und zur Abschätzung der Messunsicherheit durch, ggf. auch als Inhouse-Schulungen.
Neuigkeiten AQS
Aktuelle Tipps:
- Die LAWA AQS-Merkblätter sind jetzt auf der Webseite der LAWA kostenfrei verfügbar.
- Eurolab-D "Kochbuch"-Dokumente. Informelle Dokumente, die Laboratorien eine Hilfestellung bei der Umsetzung der Norm DIN EN ISO/IEC 17025 bieten sollen. Schauen Sie mal rein.
- Eurachem guidance documents and information leaflets on a range of issues in quality and accreditation for analytical measurement - teilweise auch auf deutsch
- Eurachem reading list - welche Dokumente sind für die Qualitätssicherung in der Analytik wichtig?
Kontaktadresse
AQS Baden-Württemberg
Institut für Siedlungswasserbau, Wassergüte- und Abfallwirtschaft
Arbeitsbereich Hydrochemie und Analytische Qualitätssicherung
Bandtäle 2
70569 Stuttgart
Deutschland
Tel.: +49(0)711 685-65446
Fax: +49(0)711 685-55446
E-Mail: info@aqsbw.de
Ihre Ansprechpartner

Michael Koch
Dr.-Ing.Wissenschaftlicher Leiter AQS Baden-Württemberg (LFL / AQS)

Frank Baumeister
Dr.-Ing.Technischer Leiter AQS
Mirela Kordic
Sekretariat AQS